First law of the electrolysis
Faraday's first law of electrolysis is states that"The mass of substance deposited,liberated is directly proportional to the passed amount of the charge to the electrolyte solution.
Mathematically it can be written as,
Explanation of the Faraday's first law of electrolysis.
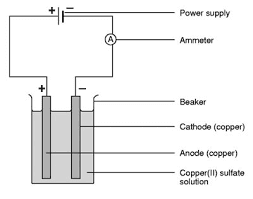
At first a cleaned and dried copper voltmeter is taken.Then cleaned cathode is kept inside the voltmeter which is weighed before kept inside.After all the arrangement,suitable amount of the current is passed to the voltmeter in time T1. After this the cathode plate is again weighed to know the mass of substance .Experiment is repeated with the same amount of current but the different time interval T2 and the cathode plate is again weighed to know the mass.Then it would be found that,
Mass of substance deposited is directly proportional to the time i,e.
M@t...........(1)
Again the experiment is repeated with the different current but for the same time.Then let I1 & I2 be the currents passed to the electrolyte for the same time interval and the corresponding masses deposited in the cathode plate.
Mass of substance deposited is directly proportional to the current passed to the electrolyte i.e.
M@I...............(2)
From the equation (i) and (ii) we have,
M@IT
M@q
Where q=it known as the charge passed to the electricity passed to the electrolyte.Hence it is the Faraday's first law of electrolysis.Hence verified.
Faraday's second law of electrolysis.
Faraday's second law of electrolysis is states that "When the same amount of current is passed to the electrolyte solution the mass of substance deposited liberated is directly proportional to their chemical equivalents.
Mathematically it can be written as,
M@E
Where M is the mass of substance deposited and E be the chemical equivalents.
Experimental verification of the second law of electrolysis.
To verify this experiment we take the three voltmeter like water voltmeter,silver voltmeter,and the copper voltmeter as shown in figure below.
When The same amount of the current is passed to these three voltmeter(water voltmeter,silver voltmeter and copper voltmeter )the mass of substance deposited in the cathode plate is directly proportional their equivalents.If M1,M2,M3, be the mass of the hydrogen,copper and silver deposited in the cathode plate.similarly E1,E2,E3 be their equivalents respectively.
Therefore on solving it is found that the mass of substance deposited is directly proportional to their equivalents.Which is the faraday's second law of the electrolysis.Hence verified.you can also learn about the Faraday's second law of electrolysis by watching this video below.
Faraday's second law of electrolysis.
Faraday's second law of electrolysis is states that "When the same amount of current is passed to the electrolyte solution the mass of substance deposited liberated is directly proportional to their chemical equivalents.
Mathematically it can be written as,
M@E
Where M is the mass of substance deposited and E be the chemical equivalents.
Experimental verification of the second law of electrolysis.
To verify this experiment we take the three voltmeter like water voltmeter,silver voltmeter,and the copper voltmeter as shown in figure below.
 |
| faraday's second law of electrolysis. |
When The same amount of the current is passed to these three voltmeter(water voltmeter,silver voltmeter and copper voltmeter )the mass of substance deposited in the cathode plate is directly proportional their equivalents.If M1,M2,M3, be the mass of the hydrogen,copper and silver deposited in the cathode plate.similarly E1,E2,E3 be their equivalents respectively.
Therefore on solving it is found that the mass of substance deposited is directly proportional to their equivalents.Which is the faraday's second law of the electrolysis.Hence verified.you can also learn about the Faraday's second law of electrolysis by watching this video below.









0 comments:
Post a Comment